Good news-high performance liquid chromatography tandem mass spectrometry and neonatal genetic and metabolic disease screening kit were approved
Inputtime:2020-03-26 10:41:14 Views:
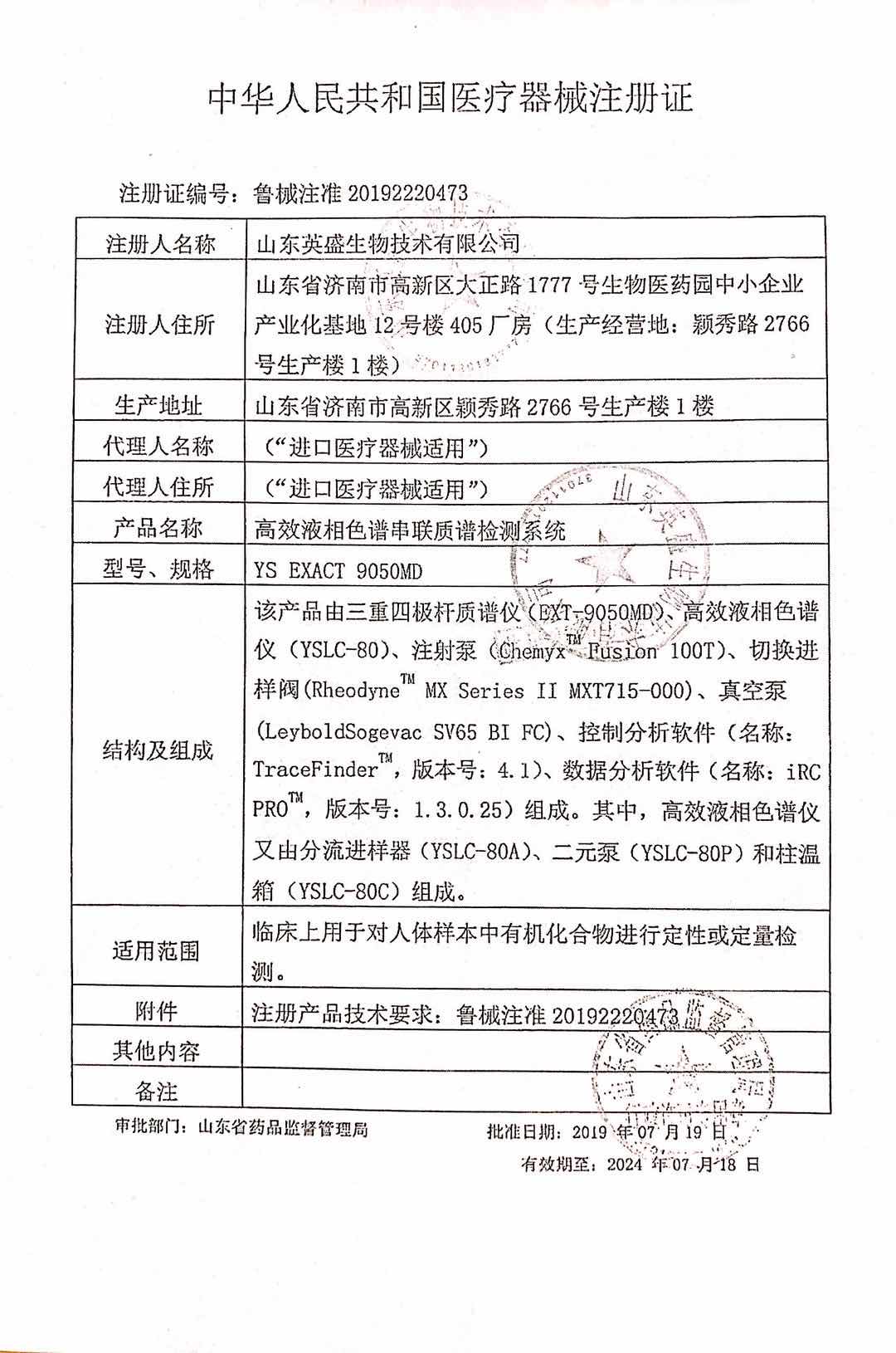
In July 2019, Shandong Yingsheng Biotechnology Co., Ltd. received two good news one after another. After the review of the State Drug Administration and Shandong Provincial Drug Administration, Yingsheng biological's neonatal genetic and metabolic disease screening kit and high performance liquid chromatography tandem mass spectrometer ys exact 9050md were officially approved.
Since then, the MS ys exact The mass spectrometry one-stop solution represented by 9050md will enter into clinical application with high precision, high throughput and high standard attitude, and will accelerate the development of process, standardization, automation and intelligence of clinical mass spectrometry test. At the same time, the approval of newborn genetic metabolic disease screening kit will officially lead the standard of newborn genetic metabolic disease detection by tandem mass spectrometry The tide of change.
Ys exact 9050md high performance liquid chromatography tandem mass spectrometer has the characteristics of extraordinary quantitative, stable performance, high sensitivity, easy to use and so on. It can be used for clinical small molecule quantitative analysis, and can detect many clinical demand projects including vitamins, genetic metabolic diseases, blood drug concentration, hormones, etc.
Yingsheng biological's neonatal genetic and metabolic disease screening kit can detect 48 kinds of genetic and metabolic diseases. It has the characteristics of high precision, high throughput, short detection time, and can meet the clinical needs in all aspects.