Individualized medication solutions
Inputtime:2020-01-15 08:31:41 Views:
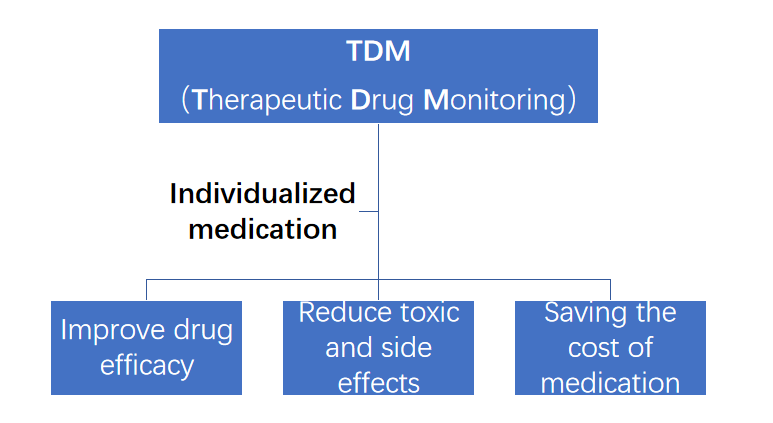
Therapeutic drug monitoring (TDM) is to determine the drug exposure, pharmacological markers or efficacy indicators in patients, use quantitative pharmacological model, take the drug treatment window as the benchmark, and develop the individualized drug delivery scheme suitable for patients. The core is individualized drug therapy

TDM development milestone
- TDM sprouts in the early 1960s
- The rise of TDM in the 1970s in Europe and the United States
- 1979: the official journal of the international society of therapeutic drug monitoring
- In the 1980s, therapeutic drug monitoring has become a widely accepted term
- In 1989, the Ministry of health listed TDM in the standard of hospital grade evaluation
- There are clear inspection standards and requirements for carrying out TDM in hospitals in the review of class III and class A hospitals in 1993
- In 2011, the professional committee of therapeutic drug monitoring and research of Chinese Pharmacological Society was established
- 2011 AgNP consensus guidelines for the monitoring of psychotherapeutic drugs
- In 2015, the clinical pharmacology group of pediatric branch of Chinese Medical Association issued the expert consensus on the monitoring of therapeutic drugs for children. Children are the special population of TDM, and the value of TDM is greater
- In 2016, vancomycin TDM guidelines developed under the leadership of TDM Research Committee of Chinese Pharmacological Society were officially included in the National Library of clinical practice guidelines of the United States
- From 1995 to 2018, 37 TDM guidelines have been published in the world, including 5 in Chinese. The relevant guidelines have played an important role in promoting and standardizing the use of TDM
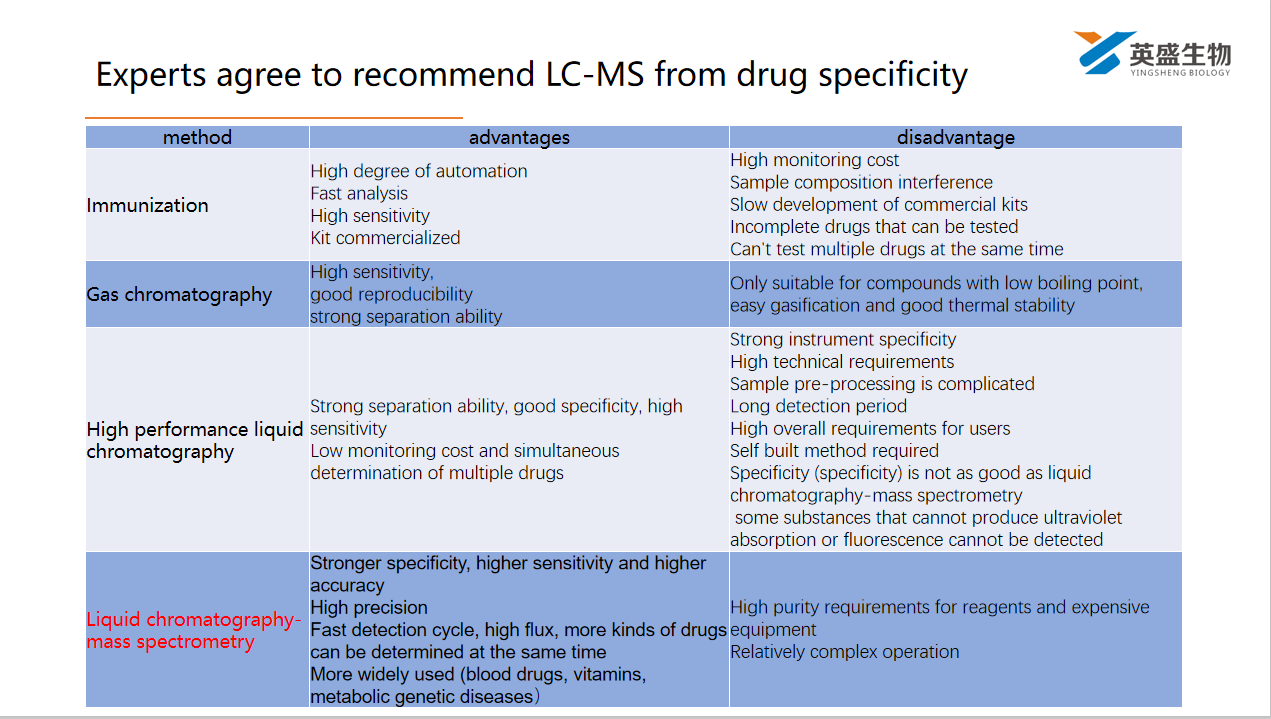
- In 2019, TDM Research Committee of Chinese Pharmacology Society formulated the expert consensus on the norms of therapeutic drug monitoring (2019 Edition), and recommended the use of liquid chromatography-mass spectrometry technology from the aspect of drug specificity (specificity)
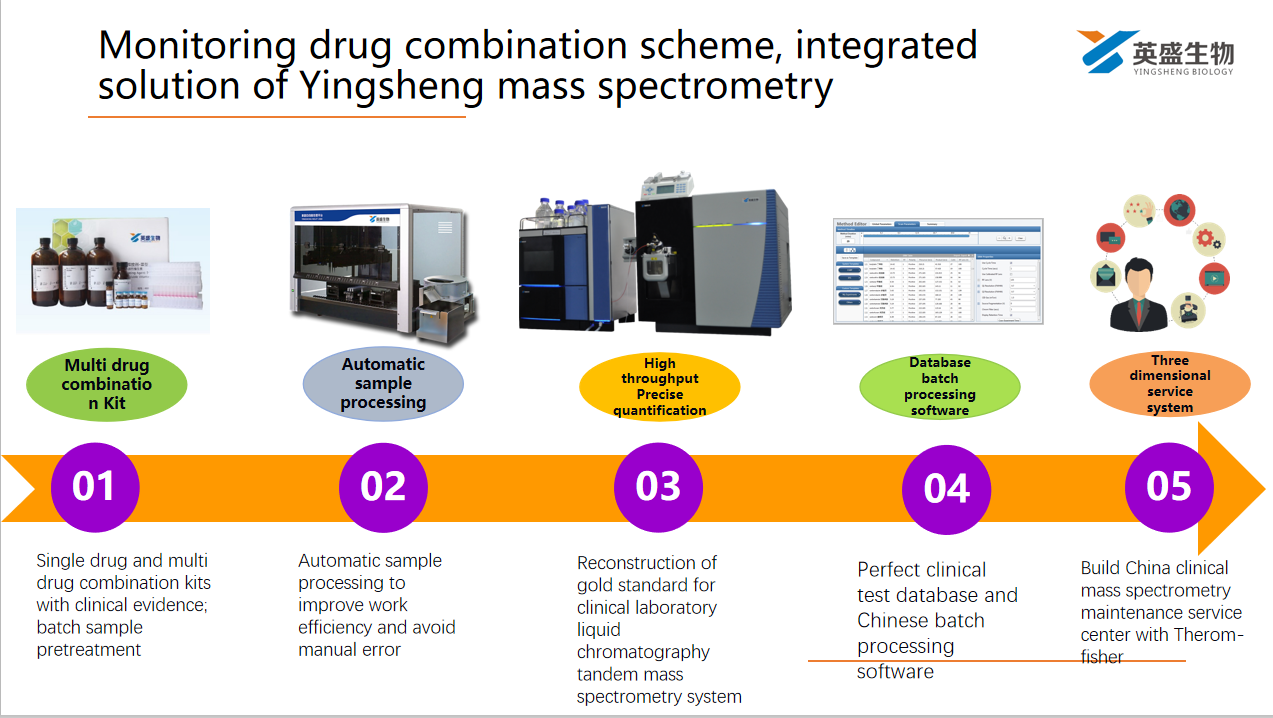
- TDM trend: from the quantitative mode of monitoring single drug compound in the past to the systematic monitoring mode of combining multi drug qualitative and quantitative parameters mainly monitoring drug treatment scheme (drug combination)
Clinical significance of TDM
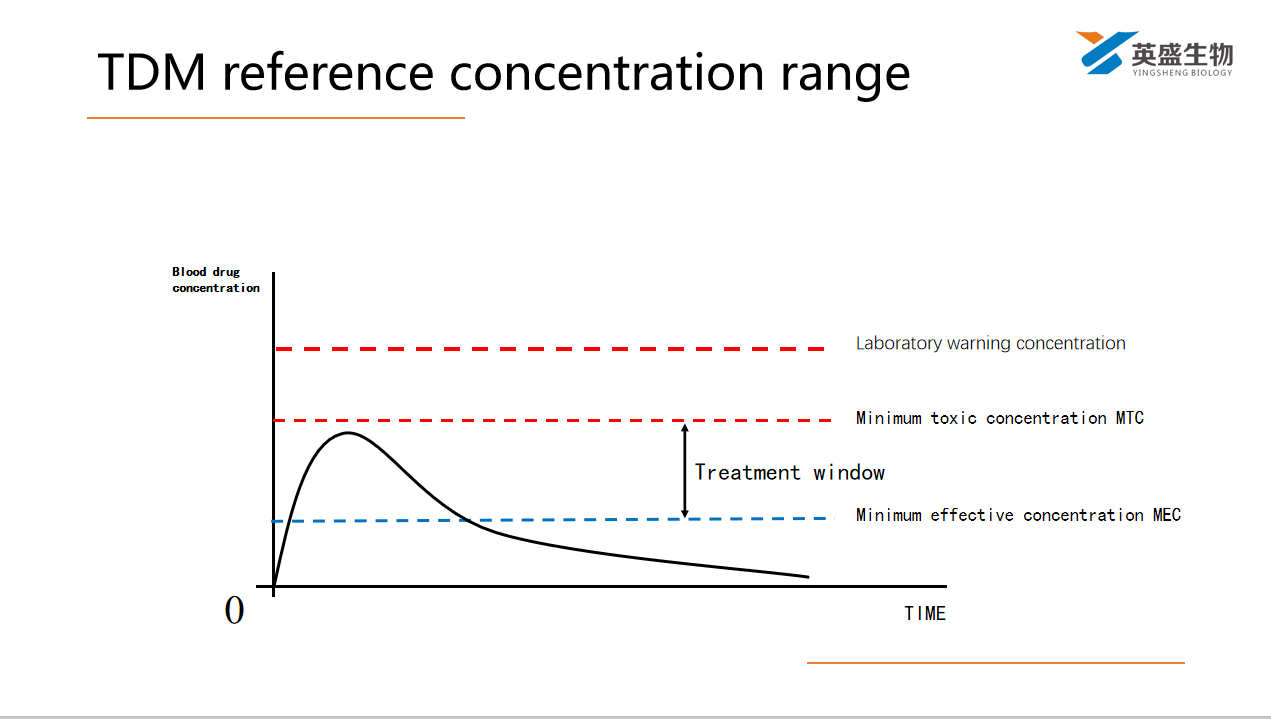
TDM sampling time: steady state concentration
-
Most of the effective concentration range of drugs is determined when the concentration of drugs in the plasma reaches or approaches the steady-state concentration (CSS). Generally, it takes 4-5 half lives to reach CSS.
-
If the treatment effect is suspected to be unsuccessful, steady-state Valley concentration is taken as an important index.
-
If toxic reactions due to excessive drug concentration are suspected, samples should be taken at peak concentration.
-
Blood should be taken at the end of the interval of administration, i.e. before the next administration.
-
Two blood samples, peak concentration and valley concentration, are often taken for the drugs with fast elimination because of the great fluctuation of blood concentration during the interval of administration.
-
For more than 90% of psychoactive drugs, the steady state can be achieved after one week of continuous administration