



With the rapid development of clinical laboratory medicine, precision medicine and other fields, the clinic is more dependent on more accurate, faster and simpler test methods and detection technologies.
Mass spectrometry is more sensitive, specific and accurate than traditional technologies, and has the unique advantages of high throughput, high efficiency and low cost. It is expected to bring new solutions for medical innovation.
1. Mass spectrometry
1.1 basic principles
Mass spectrometry USES high-speed electrons to bump gaseous molecules or atoms of the sample being analyzed, ionize them, accelerate and collimate the positive ions under the action of an electromagnetic field, and then enter the mass analyzer.
Under the action of the magnetic field of the mass analyzer, the sample ions are separated by different mass/charge ratios (m/z) and enter the detector. The ion signal is amplified and recorded on the readout device to form the corresponding mass spectrogram, and then the qualitative, structural and quantitative analysis of the material is carried out.
The mass spectrometer is generally composed of sample injection system, ion source, mass analyzer, detector and data processing system.
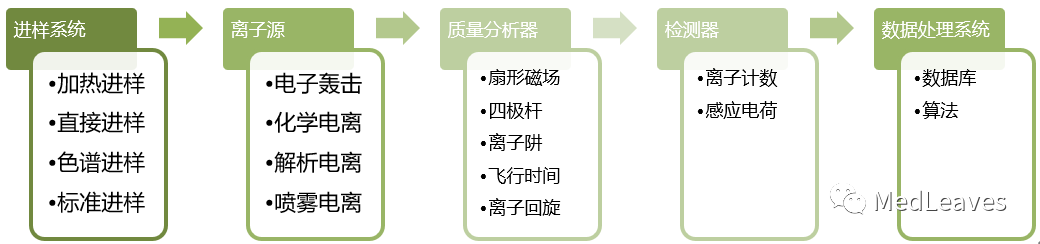
Ion source and mass analyzer are the core technologies of mass spectrometer.
1.2 basic structure
1.2.1 sampling system
Clinical samples are complex mixtures and cannot be used directly for mass spectrometry, so a suitable sampling system is needed to purify and introduce the substances to be tested.
Gas chromatography (GC) and liquid chromatography (HPLC/UPLC) are the classical methods of introduction.
In addition, supercritical fluid chromatography (SFC) and capillary electrophoresis (CE) can also be used in conjunction with mass spectrometry, greatly increasing the flexibility of sample introduction.
1.2.2 ion source
The role of ion source: one is to ionize the sample material;
The second is to extract, accelerate, focus and collimate the ions.
At present, there are many kinds of common ion sources in the market, mainly including the following:
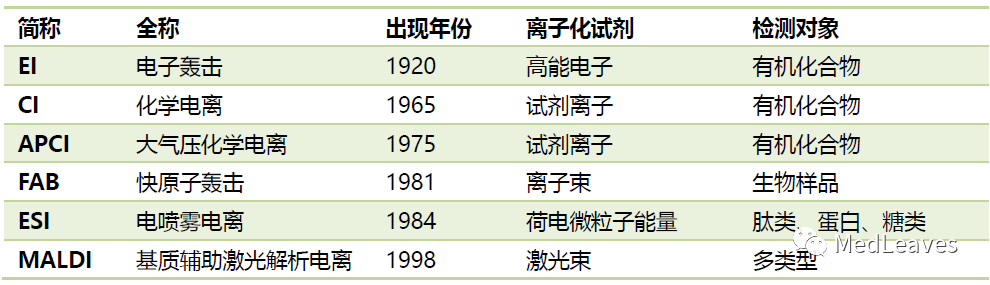
Source: zhiwang literature
1.2.3 quality analyzer
The mass analyzer is the heart of the mass spectrometer and can separate the sample ions generated in the ion source according to the mass charge ratio m/z.
There are many types, such as Quadrupole mass analyzer and Time of flight mass analyzer.
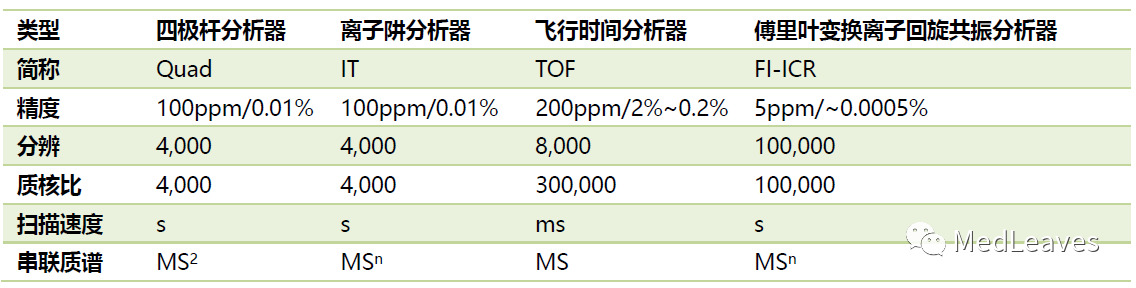
Source: cnki, public website
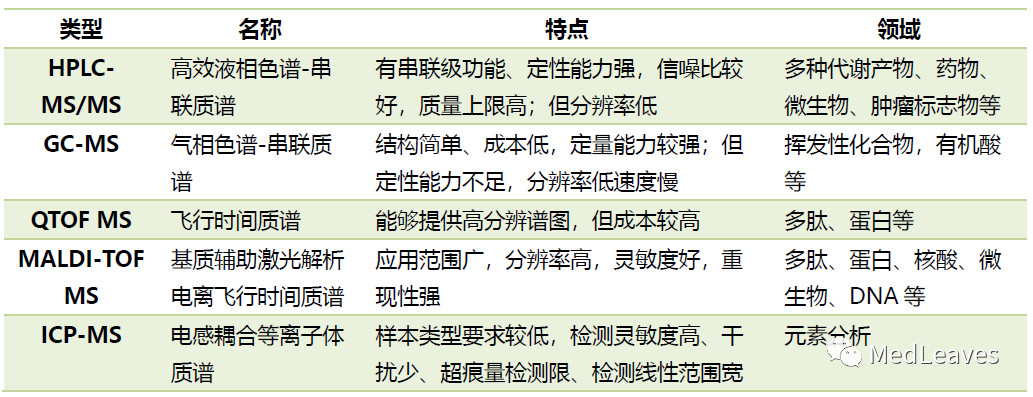
1.3 main types of clinical mass spectrometry
1.3.1 liquid chromatography-mass spectrometry (lc-ms)
LC - MS (clinical mainly HPLC/UPLC) since 2000 has become analysis of sophisticated weapons, can be widely used in all kinds of metabolites in biological samples such as blood, urine and biological molecules such as proteins, peptides, vitamins, hormones, such as analysis, is an important frontier inspection technology in clinical inspection and main reference method for the determination of biological small molecules.
It has been widely used in screening genetic and metabolic diseases, drug research, vitamin detection, hormone detection and tumor marker screening.
1.3.2 gas chromatography-mass spectrometry (gc-ms)
Gc-ms combines the advantages of gas chromatography and mass spectrometry, and has the high resolution of GC and the high sensitivity and strong identification ability of mass spectrometry.
Gc-ms can complete the separation, identification and quantification of the components to be measured at the same time, and is widely used in the separation and identification of complex components.
Gc-ms has unique advantages in screening and urine detection of genetic and metabolic diseases in newborns, and can realize "one method to detect a variety of diseases".1.3.3 inductively coupled plasma mass spectrometry (icp-ms)
Icp-ms is an inorganic element and isotope analysis test technique developed in the 1980s. It combines the characteristics of high temperature ionization of inductively coupled plasma with the advantages of rapid and sensitive scanning of mass spectrometer to form a highly sensitive analysis technique.
Icp-ms has many advantages in the field of drug detection: low requirement on sample type, high detection sensitivity, less interference, ultra-trace detection limit, wide detection linear range, etc.
1.3.4 matrix-assisted laser time-of-flight mass spectrometry (maldi-tof MS)
MALDI was first proposed by Hillenkamp and Karas in 1987. By combining this "soft ionization" technology with time-of-flight mass spectrometry (TOF), it successfully achieved the goal of providing a rapid and highly reliable detection method for biological macromolecules, and provided a new analytical method for clinical use.
Improved quantitative performance of the new generation of maldi-tof has led to its faster use in routine clinical tests, such as cancer typing directly from serum, tissue extracts and other body fluids.
Tissue imaging;
Protein modification analysis;
Identification and verification of biomarkers;
Immunoassay by mass spectrometry;
Peptide quantification;
Clinical determination of diagnostic and treatment-related biomarkers.
Source: cnki
1.4 new mass spectrometry technology and clinical application frontier
1.4.1 open atmospheric ion source mass spectrometry platform with multiple tasks
This method was presented by professor jian-tai xie of national sun yat-sen university in the meeting of the intersection of mass spectrometry and clinical mass spectrometry 2019.
It can quickly identify pesticides used by suicide victims, overdoses of drugs and toxic substances in the bodies of those poisoned by Chinese herbs.
With this method, the sample does not need to be pretreated, and the td-esi /MS or ELDI/MS sampling process of the toxin can be completed within 60 seconds.
The detection limit of pesticide, drug abuse and toxic herbal ingredients in this method is below PPM, and the experimental reproducibility is good (RSD< 15%), indicating that this method has high precision.
The results show that td-esl /MS and ELDLMS can provide timely toxicological information for emergency patients, and provide reliable reference for follow-up treatment.
1.4.2 artificial intelligence + mass spectrometry detection
David a. Herold, A professor in the department of pathology at the university of California, San Diego, said AI could be used to assess and analyze mass spectrometry information to assess disease and proteomic metabolites.
For AI, the most important thing is data quality. In the context of significant advances in HPLC, high-resolution mass spectrometry, ion migration spectroscopy and increasing MALDI ionization efficiency, mass spectrometry has been able to measure analytes more accurately and accurately, providing more and more comprehensive data.
Tissue imaging by high-resolution mass spectrometry can increase the molecular information about the tissue being examined, thus increasing the details of the information provided, and the diagnostic success rate is expected to be close to 100%.
This trend coincides with the development trend of intelligent industry and manufacturing.
2. Clinical mass spectrometry
2.1 the history
Mass spectrometry has won 13 Nobel prizes in the 100 years since Francis William Aston invented the first mass spectrometer in 1919.
At first, mass spectrometry was widely used in research institutions, and then gradually applied to pharmaceutical, food, environmental and clinical fields.
Gc-ms was first used in the 1980s to detect marijuana in the urine of U.S. navy pilots, marking the entry of mass spectrometry into the medical testing field.
In 1988, the United States federal drug testing administration issued guidelines requiring that therapeutic drug testing be confirmed by mass spectrometry, thus establishing the importance of mass spectrometry in clinical drug testing.
At the same time, with the development of "soft power" technologies such as electrospray and assisted laser analysis, the detection of biological macromolecules such as proteins and nucleic acids became possible, and the application scope of mass spectrometry in clinical detection was greatly expanded.
In the 1990s, lc-ms was tried to be applied to the screening of genetic and metabolic diseases in newborns.
At the beginning of the 21st century, maldi-tof made an appearance in the field of molecular diagnosis of infectious diseases. In 2013, the FDA approved the use of maldi-tof for identification of microorganisms for the first time.
In recent years, the demand of hospitals or third-party institutions for clinical mass spectrometry technology continues to increase, and various clinical mass spectrometry analysis platforms have been established.
In the clinical field, mass spectrometry can be applied to clinical biochemistry, clinical immunology, clinical microbiology and clinical molecular diagnosis.
2.2 application fields
2.2.1 screening of genetic and metabolic diseases in neonates
Genetic metabolic disease (IEM for short) is also known as congenital metabolic disorder (lMD).
There are more than 500 kinds of common diseases of genetic metabolism, and the incidence of single diseases of genetic metabolism is relatively low, but the overall incidence can reach 0.2% of live births.
IEM is easy to be ignored. Children with IEM have no special clinical manifestations in the early stage, and there are many cases of neglect, misdiagnosis, missed diagnosis or hard to confirm. Once the symptoms of IEM appear, the damage to children will be hard to reverse.
However, most of the IEM is treatable and controllable, but only if timely and accurate birth screening and early treatment are performed.
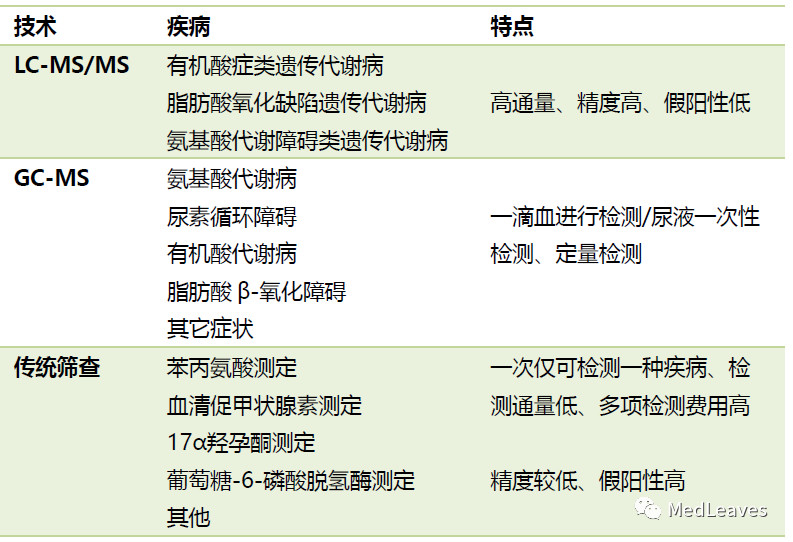
Source: notson's test
2.2.2 vitamin D detection
Vitamin D is a fat-soluble steroid that is essential for bone formation and function.
Long-term vitamin D deficiency can lead to rickets, osteoporosis, osteomalacia and other diseases. In recent years, many studies have reported that vitamin D deficiency is associated with some complications such as tumor, hypertension and diabetes.
Regular detection of vitamin D levels is of great significance for disease prevention. Some European and American countries have included vitamin D in routine physical examination, and vitamin D examination is also a routine part of pregnancy examination.
The determination of 25(0H) D (including D2 and D3) in serum is the best indicator for evaluating vitamin D in human body.
Traditional methods have poor specificity and anti-matrix interference ability. Tandem mass spectrometry can accurately determine the concentrations of 25(0H)D2 and 25(0H)D3 at the same time, which is recognized as the gold standard for the detection of 250HD.
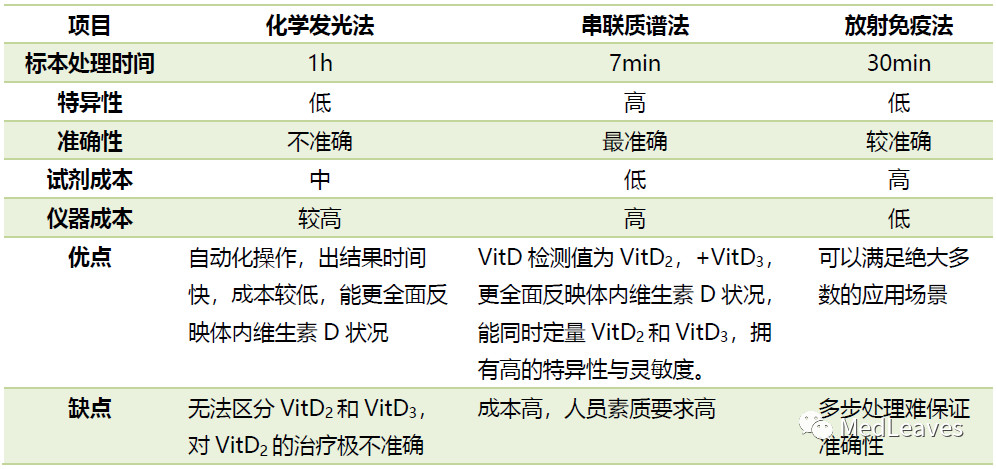 Source: laboratory medicine network
Source: laboratory medicine network
2.2.3 microbial detection
The analysis method of microbiological detection data by mass spectrometry has obvious advantages. It is usually used to assist diagnosis, determine the type of infection and guide drug use in clinical practice.
There are 7 categories of routine clinical microbiological testing programs in Chinese medical institutions, 152 of which mainly include eight categories: fungi, actinomycetes, spirochetes, bacteria, rickets, chlamydia, viruses and mycoplasma.
There were 81 bacterial tests, accounting for 52.5%.
At present, most of the test products used in microbiology laboratories are based on traditional methods (staining, culture and non-culture), which have many problems: long sample culture time, limited identification species and complex manual intervention process.

With mass spectrometry (MALDI - TOF MS) identification of microbial main principle is to use a database of known species, through microbial protein spectrum detection, because of the different species ribosomal protein (2 ~ 20 kda) there are differences in size, the spectra obtained will be against a database of microorganism reference map matching results can be obtained after identification.
Main features: fast speed, less links and less investment.
Database is the core barrier of microbial mass spectrometry, the quality and quantity of the maps in the database will directly affect the success rate and accuracy of the identification.
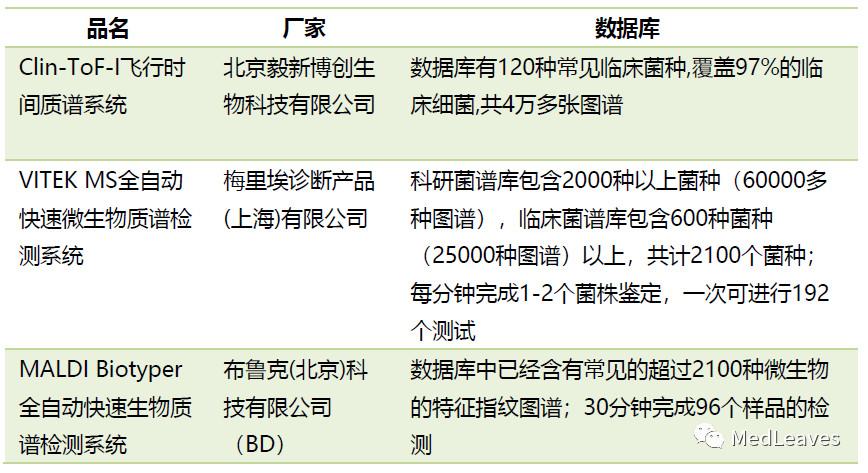
Source: company official website, public data
2.2.4 drug testing
Therapeutic drug monitoring (TDM), also known as therapeutic drug detection, refers to the collection of blood drug concentration (or urine, saliva) while observing the efficacy of drugs during clinical drug therapy, and the optimization of drug administration plan in combination with pharmacokinetics and pharmacodynamics to achieve satisfactory efficacy and avoid toxic and side effects.
There are many methods of traditional drug detection, and mass spectrometry is the most accurate method.
The development of TDM in China is still in the early stage. The existing clinical guidelines can be used for reference, including 128 psychotropic drugs defined in the 2011 consensus guidelines for the monitoring of AGNP psychiatric drugs: 2011.
In 2015, Chinese medical association clinical pharmacology group "children's therapeutic drug monitoring expert consensus" identified 15 drugs.
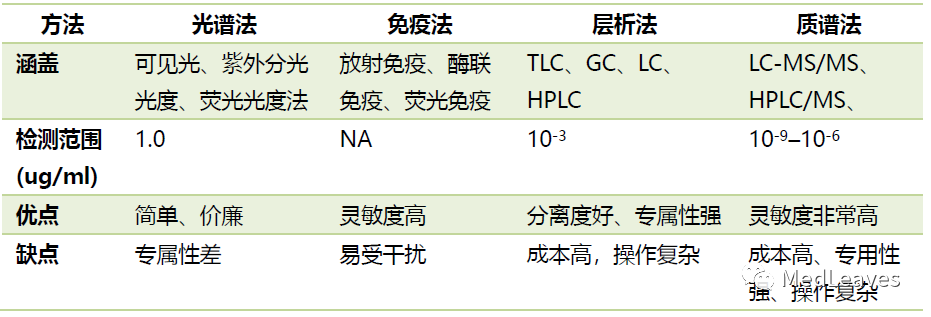
Source: lilac garden
3. Market forecasts
3.1 mass spectrometer - national strategic emerging industry
3.1.1 market size
On the international side, according to the statistics and research on mass spectrometry market conducted by many research and consulting companies worldwide, it can be predicted that the global mass spectrometer market scale will be about usd 7 billion by 2021.
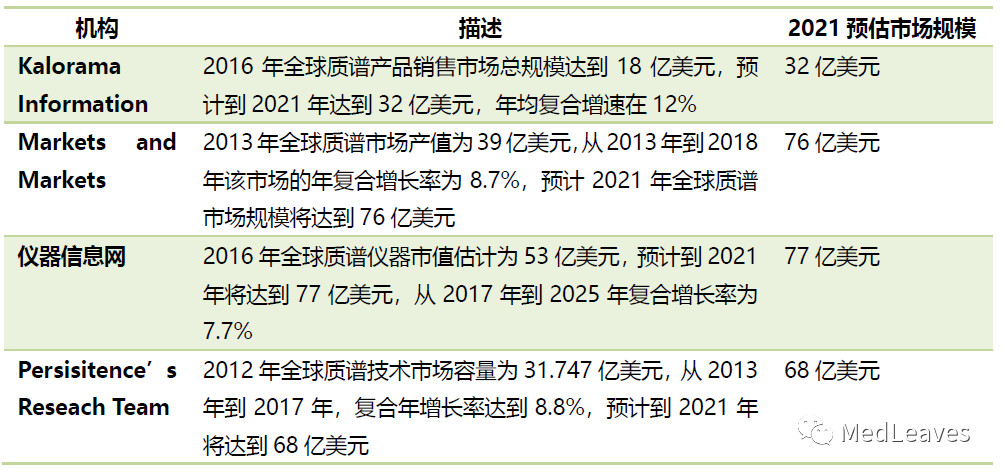
Data source: probe capital, official website data
Domestically, the market is expected to reach 23.5 billion yuan in 2026.
According to the data from the national health and family planning commission by the end of June 2017, there are 2,286 tertiary hospitals and 8,118 secondary hospitals in China. If each secondary hospital or above is required to be equipped with at least one clinical mass spectrometer at the unit price of 1 million yuan, the total market value of clinical mass spectrometer in China will reach 10.4 billion yuan.
If calculated conservatively based on the average annual compound growth rate of the international market of 7.70%, the market size of mass spectrometer in China will reach at least 23.5 billion yuan in 2026.
The technical barriers of mass spectrometry are relatively high.
According to Chinese customs statistics, China imported 12,426 mass spectrometers in 2018, with imports from the United States, Germany, Singapore and Japan accounting for more than 80 percent.
The import amount of mass spectrometer in China shows an overall trend of increasing year by year, increasing from 4.468 billion yuan in 2014 to 9.577 billion yuan in 2018, with an average annual compound growth rate of 21.00%.
Domestic market has larger imagination space.
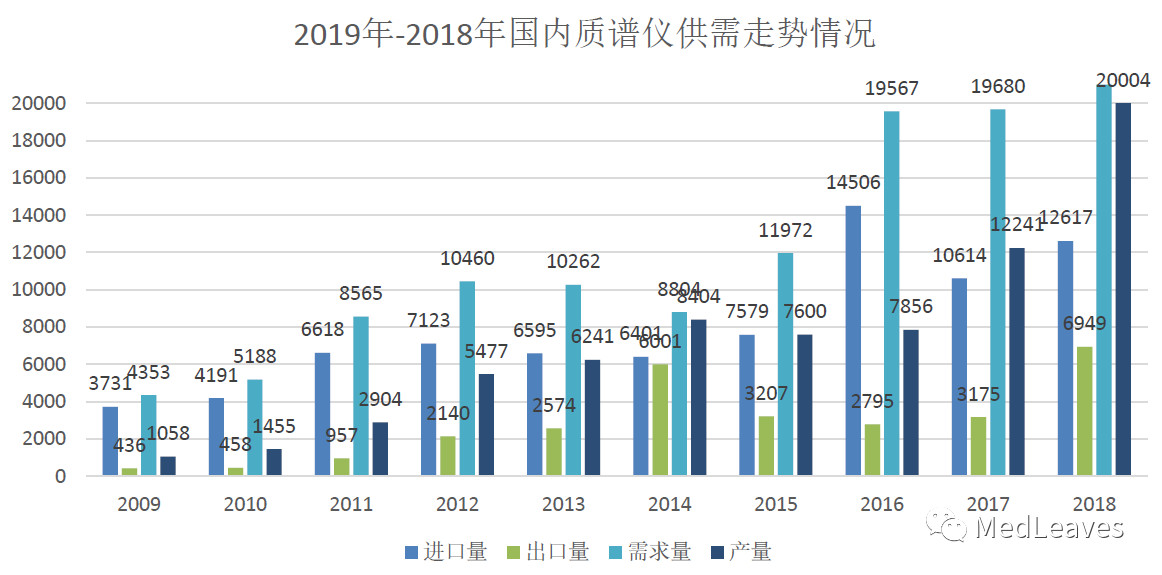
3.1.2 market pattern
From the number of patents owned, the head of the enterprise mainly accumulated in the United States and Japan.
The United States and Japan are the main source countries of the new mass spectrometer technology.
As of 2016, the United States ranked first with 1,511 patents, accounting for 35.45% of the total in the last two decades.
Japan ranked second with 1,156 patents, accounting for 27.12%;
China ranked third with 638 patents, accounting for 14.97%;
Britain ranked fourth with 318 patents, and Germany fifth with 170.
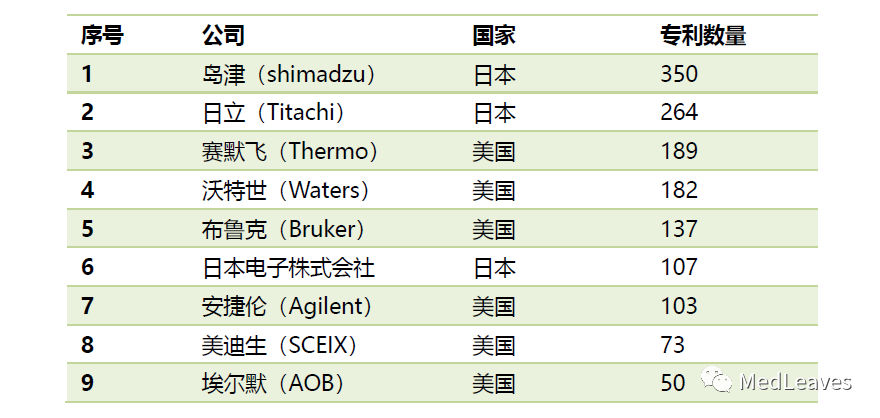
Data source: cnki, data intelligence journal
From the perspective of market share, the mass spectrometer market basically presents an oligopoly situation.
The four leading traditional analytical instrument manufacturers account for more than 70% of the mass spectrometer market, and danaher subsidiary SCIEX ranked first with 22% market share in 2016.
Agilent, syme and brook have 20%, 17% and 16% market shares, respectively.
Waters and shimadzu (meriere) also had 13 and 10 percent, respectively.
3.2 clinical mass spectrometry - 10 billion blue ocean market
3.2.1 market overview
According to the research data of zhongtai securities, clinical mass spectrometry in the United States accounts for about 15% of the total medical detection market. Meanwhile, according to the revenue structure of Quest, about 1 billion of the 7.5 billion us dollars in 2016 came from the mass spectrometry program, and the mass spectrometry accounted for about 13%. Therefore, the total clinical mass spectrometry market in the United States is about 9 billion us dollars.
The domestic market has great development potential.
In developed countries in Europe and the United States, there are more than 400 mass spectrometry testing items serving clinical diagnosis and treatment. Compared with the United States, China's mass spectrometry clinical testing is in the early stage of development.
According to carrey consulting, China's mid-range clinical mass spectrometry market currently accounts for less than 1% of the medical testing market, far less than the 15% in the United States.
Therefore, it is predicted that the market size of China's medical detection terminal is about 200 billion yuan, and the potential market of clinical mass spectrometry is about 30 billion yuan.
The development of clinical mass spectrometry in foreign countries has only been more than 10 years, and it is still in the period of rapid development. Therefore, the domestic market of long-term clinical mass spectrometry still has a large growth space.
3.2.2 key markets
3.2.2.1 mass spectrometry detection of genetic and metabolic diseases of neonates
It is estimated that in 2025, the screening market for genetic and metabolic diseases of neonates by mass spectrometry is RMB 1.764 billion.
Based on the estimation of 15 million newborns in 2018, the average price of mass spectrometry detection is 250 yuan, the overall screening rate of 85%, and the mass spectrometry penetration rate of 20%, the market of 2018 is 640 million yuan.
If the screening rate increases to 98%, mass spectrometry penetration increases to 60%, and the price drops to 200 yuan per time by 2025, the market space will reach 1.76 billion yuan in 2025.
Considering the continuing impact of the two-child policy, the market is expected to be larger than expected in the future.
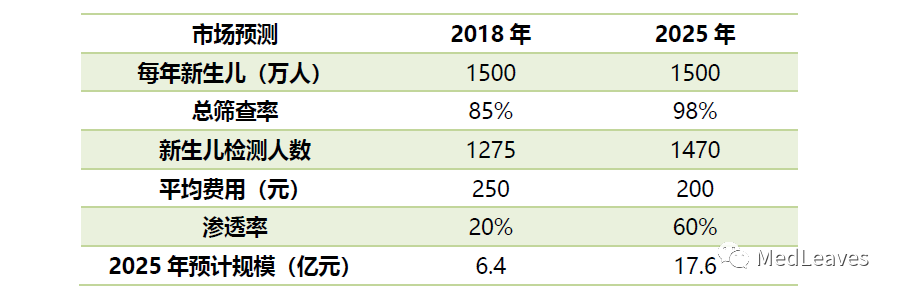
Data: national bureau of statistics, western securities, cicc research department
3.2.2.2 vitamin D detection
It is estimated that the market space of vitamin D mass spectrometry will exceed 7.6 billion yuan in 2025.
People who usually need vitamin D testing include pregnant women, newborns and people over the age of 60.
Suppose that by 2025, the number of pregnant women is 20 million, the penetration rate is 40%, and the annual examination is 4 times.
The number of newborns was 18 million, with annual examination.
There are 230 million people over 60 years old and the penetration rate is 5%. Assuming that the cost of vitamin D testing is 150 yuan/person, the market space will reach 7.35 billion yuan by 2025.

Data sources: national bureau of statistics, China international capital research, western securities
3.2.2.3 microbial diagnosis
The market for microbial diagnostics is expected to reach 5.7 billion yuan in 2025.
Assuming that mass spectrometry penetration rate reaches 30% and the microbial testing market maintains a compound growth rate of 3% with the encouragement of mass spectrometry by national policies by 2025, and assuming that both the mass spectrometry and the traditional biochemical terminal prices drop by 20%, the microbial mass spectrometry testing market can reach 5.67 billion yuan in 2025.
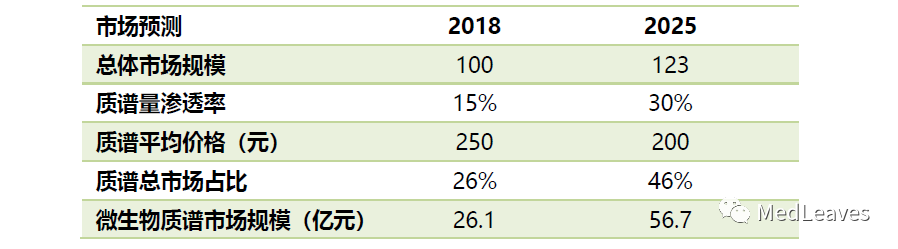
Source: gotejia, western securities, cicc research
4. Enterprise research
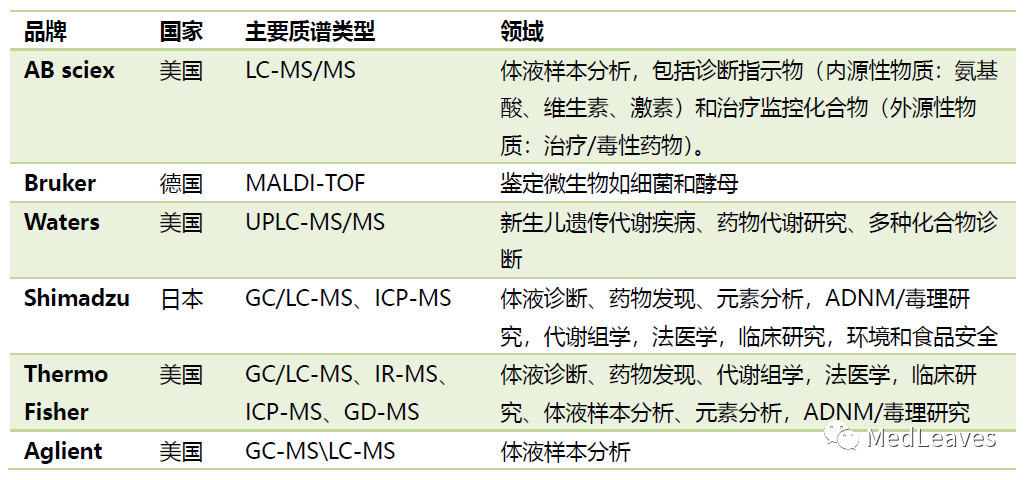
Clinical spectrum main brands of the market, currently include international brand agilent (Aglient), Dana Hector (AB SCIEX), perkin Elmer (PerkinElmer), the game's fly the er, brooke cooperation agency (BD), walter (Waters), such as from shimadzu, domestic brands are shandong ying sheng, tianrui apparatus and RongZhi biology, zhejiang, wo believe instrument, guangzhou rui, antu biology, yi bo gen and cheng's new di, RongZhi, guangzhou forword, and so on.
4.1 international giants
4.1.1 Aglient
Agilent (Agilent) was founded in 1999, headquartered in Santa Clara, California, USA.
The company is committed to the life sciences, diagnostics and applied chemistry markets, providing instruments, services, consumables, applications and expertise to customers worldwide.
Agilent is a global leader in analytical laboratory technology with over 40 years of expertise in the field of mass spectrometry. In addition to mass spectrometry systems, Agilent provides technical support for ion sources, databases and spectrum libraries, and application packages.
Agilent's clinical mass spectrometry products are mainly GC/MS series, LC/MS series and high-throughput screening systems.
Its GC/MS series, such as 5977B GC/MSD, are used for the determination of carbonate solvents and additives in lithium battery electrolyte.
7000D for the determination of a variety of pesticide residues.
LC/MS series, such as 6495B LC/TQ, can be used for the analysis of high quality charge ratio polypeptide ions.
6230B can be used for qualitative metabolic flow analysis of 13c-glutamine.
LC/MSD can be used for n-glycosylation analysis of therapeutic proteins.
High-throughput screening systems such as Rapidfire 360 are suitable for whole-protein and fragmentation analysis, drug-drug interactions, CYP inhibition, metabolic stability, Pgp inhibition (digoxin), plasma protein binding rate determination, permeability (caco-2 and PAMPA), and complex sample analysis
4.1.2 Thermo Fisher Scientific
Thermo Fisher Scientific, formerly Thermo Electron Corporation, was founded in 1956. Headquartered in Massachusetts, USA, Thermo Fisher Scientific is a leading global Scientific services company serving customers in pharmaceutical and biotechnology companies, hospitals and clinical fields, and has long been a leader in the instrumentation industry.
The company has several series of mass spectrometry products: liquid/gas chromatography mass spectrometry, isotope ratio mass spectrometry (ir-ms), gd-ms, inductively coupled plasma mass spectrometry (icp-ms), ion chromatography-mass spectrometry (ic-ms).
TargetQuan 3 software, QtegraTM Intelligent Scientific Data SolutionTM software and ScreenIDTM HRAMTM lc-ms system for forensic toxicology and clinical research.
In 2018, syme launched ISQ 7000 single quadrupole gc-ms, Orbitrap id-x triple high resolution mass spectrometer, TSQ Fortis triple quadrupole mass spectrometer and many other products.
4.1.3 danaher (AB SCIEX)
AB SCIEX was founded in 1970 by Dr. Barry France and others in the department of aeronautics and astronautics at the university of Toronto.
As a life sciences company, SCIEX has been a leader in tandem mass spectrometry by focusing on cutting-edge instruments, workflow solutions and supporting innovation for 40 years.
In clinical diagnosis, SCIEX has Citrine MS/MS system to measure low levels of biomarkers and metabolites.
Jasper HPLC system for separating samples with mass spectrometry;
4500 MD provides high sensitivity and high speed data acquisition for clinical diagnosis.
In addition to developing and launching mass spectrometers, SCIEX partnered with Illumina in 2014 to launch OneOmics, which aims to create the world's first multi-cluster cloud computing environment.
It integrates the next generation of proteomics (NGP) and next generation sequencing (NGS) tools based on SWATH technology.
As part of this initiative, Illumina's app store BaseSpace will host AB Sciex's proteomics cloud tools, making BaseSpace a one-stop center for genomics and proteomics "big data."
4.1.4 Bruker
Founded in 1960, Bruker is headquartered in karlsruhe, Germany, and Madison, USA.
Bruker is committed to providing analytical and diagnostic solutions for the life sciences and is a provider of high-performance systems for clinical research.
Brook, one of the world's leading analytical instrument companies, launched the first commercial maldi-tof mass spectrometer in 1992.
Brook's latest maldi-tof and TOF/TOF MS mass spectrometer, a powerful tandem mass spectrometer developed as a method for deep protein analysis, is renowned for its ultra-high performance, stability and innovative design.
Mass spectrometers are one of its main product lines, such as Trapped Ion Mobilit (TIMS), a 10kHz scanning laser for maldi-ms imaging (providing true pixel fidelity (10um spatial resolution)), and a small mass spectrometer, spotOn.
Bruker also provides software solutions such as the SCiLs laboratory for MS imaging, the fda-approved rapid identification system for MALDI Biotyper microorganisms, Biopharma Compass and Toxtyper forensic toxicology solutions.
4.1.5 Waters
Founded in 1905 and headquartered in milford, Massachusetts, Waters is a member of the standard & poor's 500-stock index.
The company is committed to the design, manufacture and sales of chromatography, mass spectrometry, thermal analyzer and rheometer and other related products, and provide related services.
Waterworld mass spectrometry (WMS) products are favored by scientists all over the world. It has been in the industry for more than 50 years and has become one of the largest companies in the analytical instrument industry.
As a pioneer of liquid chromatography-mass spectrometry (lc-ms/MS), waterworld started its clinical application in the early 1990s.
To provide a complete range of solutions for clinical testing.
Its ultra-high performance liquid chromatography tandem triple quadruple pole mass spectrometry system (uplc-ms/MS) has obtained the medical device registration certificate approved by China state food and drug administration.
The system can be used to analyze a variety of compounds, including diagnostic indicators and therapeutic monitoring compounds. It can be used to carry out screening of genetic and metabolic diseases in neonates, detection of internal exosomes, monitoring of therapeutic drugs, vitamin detection, full-spectrum amino acid analysis, poisoning cause screening, biomarker analysis and other clinical projects.
4.1.6 shimadzu
Shimadzu was founded in 1875 and is headquartered in nakaishan district, Kyoto, Japan.
Mass spectrometry is one of Shimadzu's nine core technologies. The MALDII technique, which won the 2002 Nobel Prize in chemistry, was developed by Koichi Tanaka of Shimadzu.
The company has gas chromatography-mass spectrometry, liquid chromatography-mass spectrometry series of many products.
As a famous manufacturer of test instruments, medical devices and industrial equipment, jimazu has been continuously increasing its investment in mass spectrometry market in recent years, and has successively launched several new products of UFMS series such as four-pole time-of-flight mass spectrometry lcms-9030, high-sensitivity triple-quadrupole gcms-tq8050 and icpms-2030.
In addition, software solutions such as Strain Solution, MALDI Solution and other high-precision bacterial identification software corresponding to AXIMA microbial identification system were developed.
4.2 domestic emerging
4.2.1 yingsheng biology
Founded in 2009, yingsheng biotechnology co., ltd. is located in jinan high-tech development zone. It has built a gene detection platform and a mass spectrometry detection platform around genomics and mass spectrometry, which can cover such fields as birth defect screening, disease prevention and human health services.
In July 2018, China signed a strategic cooperation agreement with thermo fisher technology co., LTD., through which lc-ms, icp-ms and other high-end mass spectrometers will be produced in China to accelerate the rise of domestic high-end medical equipment manufacturing.
In June 2019, the company partnered with Biotage AB of Sweden to provide a complete solution from instrument, consumables to technology for the clinical mass spectrometry market.
Yingsheng's product line includes 45 genetic and metabolic disease screening reagents for newborns, 14 vitamin detection reagents, automatic intelligent processing platform for mass spectrometry, and ultra-high performance liquid chromatography tandem mass spectrometry detection system developed independently, all of which have reached the international leading level.
Several products of the company have obtained or are about to obtain the NMPA registration certificate, covering all aspects of mass spectrometry testing.
Up to now, the company has obtained dozens of national patents and software Copyrights, and established 3 industry standards and 1 national standard.
Will become the leader of the mass spectrometry diagnosis track.
4.2.2 hexin instrument
Established in 2004, hexin instrument is a state-level torch key high-tech enterprise and a national "thousand talents plan" entrepreneurial enterprise, which is dedicated to the research, development, manufacturing, sales and technical services of mass spectrometer instruments in the whole field (environmental monitoring, meteorology, industrial production, medicine, etc.).
With continuous development and innovation, the company has the core mass spectrometry technology, a full set of assembly process and independent production line, and is the first in China to realize independent forward development of mass spectrometer products.
Based on years of product and technology reserves, hexin has been gradually expanding into the medical and health field in recent years.
At present, the company's microbial mass spectrometry detection system has submitted an application for special approval of the second category of innovative medical devices. Hexin instruments has two types of atmospheric pressure ionization time-of-flight mass spectrometer (api-tofms) and CMI 1600 microbial identification mass spectrometer.
Api-tofms can be used in food, medicine, protein analysis and other fields;
The CMI 1600 is a matrix-assisted laser desorption ionization time-of-flight mass spectrometer (maldi-tof MS), which is especially suitable for mixed biological samples. It can directly analyze proteins, peptides, DNA and other biological macromolecules, and it is also more safe.
4.2.3 yi xin bo chuang
Founded in 2011 and located in Beijing economic and technological development zone, yixin bochuang is the first domestic r & d and production enterprise focusing on clinical flight mass spectrometry, headquartered in zhongguancun life science park.
In June 2014, the clin-tof mass spectrometer independently developed by yixin bochuang has passed the certification of European Union CEIVD and medical device certification of China food and drug administration (CFDA).
As the first clinical time-of-flight mass spectrometry company to obtain CFDA registration certificate in China, yixin is the first company in the world to achieve single-plane multi-platform. One machine can meet the needs of clinical application in four fields of microorganism, protein, gene and sugar, and its technology has reached the international leading level.
In early 2019, clin-icp-qms-i independently developed by the company also passed the CFDA certification, which is the only icp-ms trace element analysis system in China that has obtained the CFDA registration certification.
Up to now, only two foreign enterprises in China, meriere and BD, and the microbiological mass spectrometry of yixin bochuang, have been approved by CFDA.
Yi new products and business including PacBio RS II three generation sequencing platform, platform MassARRAY nucleic acid mass spectrometry, Q Exactive protein group identification and Clin - TOF mass spectrometry platform polypeptide fingerprint identification platform, focus on genetic testing of eugenic and superior nurture, individualized health rapid detection of genetic testing, infection and infectious diseases, disease peptide marker detection, etc.
At the same time, as a partner of Nature Publishing Group (NPG) in Asia Pacific, we provide one-stop research services for clients and partners from the design of scientific research plans to the publication of SCI papers.
4.2.4 intelligent organisms
Rongzhi biological was founded in 2013, located in Qingdao, by senior mass spectrometry research and development experts, is specialized in the life science analysis equipment, consumables and solutions of the research and development, production, sales, service of national high-tech enterprises.
The company has the Quan TOF (a new generation of matrix-assisted laser desorption ionization time-of-flight mass spectrometry: moldi-tof-ms), which has been recognized by the accreditation committee composed of academicians of the Chinese academy of sciences and Chinese academy of sciences as "the overall performance has reached the international leading level".
RongZhi biological platform developed based on mass spectrometry technology with rapid identification mass spectrometry system system, quantitative analysis of protein, nucleic acid mass spectrometry mass spectrometry system, compose a quality, and the like, the food traceability system mass spectrometry system and rapid detection of foodborne pathogenic bacteria system, etc. Series of products, application of clinical medical, inspection and quarantine, food safety, CDC, and other fields.
The company has obtained 15 CFDA filing certificates for category I medical device products, 1 CFDA registration certificate for category ii medical device products, 1 medical device production license, and 2 CFDA filing certificates for category I medical device production.
4.2.5 bohui innovation
Bohui innovation was founded in 2001, the headquarters is located in Beijing zhongguancun life science park, is a committed to the clinical test products of intelligent, integrated and easy to operate biomedical high-tech enterprises, mainly engaged in clinical testing rapid detection technology research and development and application of the development, production and sales of products.
The company has a strong independent research and development strength, has formed the molecular diagnosis, immune diagnosis, atomic absorption and atomic fluorescence and mass spectrum detection five technology platform, and successfully realize the industrialization of the technology platform products, including the human body trace element quality spectrum detection system has been widely applied in the national more than 7000 hospital, detection capacity up to 22 million people a year.
The company acquired Advion in 2015 to focus on the application of mass spectrometry in the field of life sciences and provide customers with excellent mass spectrometry products and experience.
The company acquired hebei daan pharmaceutical co., ltd. and guangdong weilun bio-pharmaceutical co., ltd. to enter the bio-pharmaceutical field and further improve the health ecological industrial chain.
The company has undertaken a number of national-level major science and technology industrialization projects, including the 13th five-year major scientific instrument project "new atomic fluorescence spectrometer development and application" and so on.
4.2.6 beichen medical
Zhejiang baichen medical technology co., LTD., founded in 2015 and headquartered in hangzhou, is a third-party testing service enterprise focusing on mass spectrometry applications and innovation.
The company is relatively young, and based on the mass spectrometer of waterworld, it provides the mass spectrometry detection reagent combination service. Its early business includes the screening of postpartum metabolic diseases, the detection of endocrine diseases, the early diagnosis of tumor diseases and other clinical diseases.
The company is also involved in biological information, drug development and other third-party testing projects such as food safety testing, illegal drug monitoring and other businesses.
The company has a world-class technical team, whose members (including 5 overseas returnees with PHDS) have published more than 150 academic papers in internationally renowned journals such as Science, Nature and Nature Genetics, and hold a number of core patents in the field of clinical diagnosis.
The company will set up experimental bases in major first-tier cities, and its sales network will spread throughout the country.
The first-phase laboratory covers an area of more than 2,000 square meters and is a high-tech candidate for overseas investment and introduction of enterprises in hangzhou, "xihu district 325 plan", "hangzhou 521 plan" and so on.
4.2.7 carry out clinical mass spectrometry business in other enterprises

5. Conclusion
6. Clinical mass spectrometry has been evaluated by the industry as the "gene sequencing" of the next diagnostic field.
The growth path of mass spectrometry is the same as that of gene sequencing, which is gradually moving from scientific research to clinical practice. As an emerging technology, it has been widely expected by various fields. It is likely to replicate the development path of gene sequencing.
In China, the clinical application of mass spectrometry technology is still in the initial stage, and there are only a limited number of hospitals and institutions that carry out related technologies, as well as a relatively limited number of testing projects. Its application breadth and depth are far less than those in the United States, Japan, Europe and other countries.
The main reasons are the lag of industrial policy, the limitation of mass spectrometry technology, the lack of quality management and the confusion of industrial standards.
With the development of domestic talents and technology, the gradual maturity of market promotion and the sound of the policy and legal system, the domestic market will gradually break the silence of the monopoly of international giants, in the future, domestic enterprises are expected to achieve curve overtaking.
The resources
Meng J. Clinical application of tandem mass spectrometry in the detection of genetic and metabolic diseases [J]. World latest medical information digest, 209,19 (94) : 30-31. (in Chinese)
Wang J. The value of tandem mass spectrometry in screening for genetic and metabolic diseases in neonates [J]. Chinese medical guidelines, 202,17 (31) : 86. (in Chinese with English abstract)
[3] cao kehao, wu wenying, ge yongqi. Patent and industry development analysis of microbe clinical detection by matrix-assisted laser desorption ionization time-of-flight mass spectrometry [J]. International journal of laboratory medicine, 2019,40 (18) : 2271-2275.
Yan Victoria Zhang, Thomas Jackson. Application of mass spectrometry in clinical laboratory [J]. Laboratory medicine, 209,34 (01) : 1-7. (in Chinese)
Yin bing. Principle of mass spectrometry and its application in customs testing [J]. Science and technology information, 2010 (19) : 45 + 20.
[6] China mass spectrometry market, 16 domestic and foreign brands: meriere, brook, shimazu, waterworld, in vitro diagnostic network
[7] China's mass spectrometer demand reached 25,672 units in 2018, and domestic mass spectrometer manufacturers are catching up, China industry information network
[8] China mass spectrometry 30 billion blue sea, 27 domestic and foreign brands: syme fly, meriere, shimazu, antu... inventory!
Wisdom bank capital
[9] analysis of neonatal disease screening coverage in China from 2006 to 2016, maternal and child health in China, issue 16, 2018
[10] clinical mass spectrometry: another "gene sequencing" in the field of diagnosis, zhongtai securities, 20171212
[11] mass spectrometry: the next billion blue ocean for clinical testing, cicc
[12] the ranking of the TOP20 instrument companies in 2018 was released, sohu network, experiment and analysis
[13] the clinical mass spectrometry market will grow at a rate of 7.6% in the next five years, instrument information network, 2019.11.26
[14] China clinical mass spectrometry industry development trend forecast report 2019-2025, China industry research institute, February 2019
[15] instrument network, industry information, how has the field of mass spectrometry developed in recent decades?
What do we expect in the future?
[16] the analysis report: clinical examination on the application of mass spectrometry in the European and American countries, https://m.antpedia.com/news/1271825.html
[17] a series of reports on precision medicine, mass spectrometry industry development and key enterprises in the in-depth research of precision medicine industry, kamal consulting, August 2019
[18] in-depth analysis of mass spectrometer industry, translational medicine network (public number)
[20] clinical mass spectrometry in the medical device industry: another "gene sequencing" in the diagnostic field - 20171212-zhongtai securities
[21] hexin instrument, a new "little giant" specialized in mass spectrometry industry - 20191226 - China merchants securities